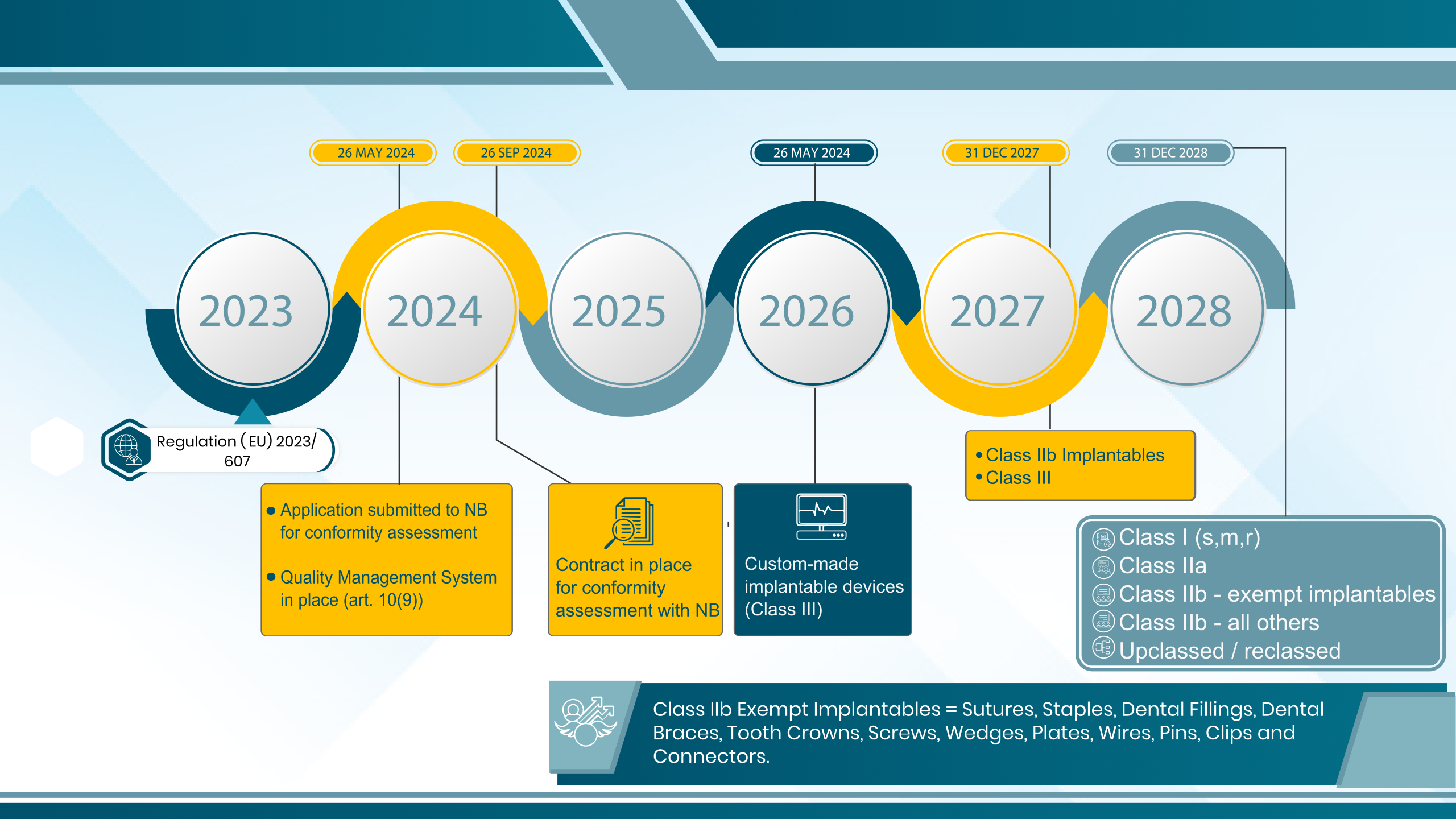
MDR Transition Timeline Extension
26th May, 2021
All new devices entering EU market need to be MDR compliant.
26th May, 2022
2 year clock to enter data in to EUDAMED begins.
26th May, 2024
MDD certificates expire.
-OR-
Application submitted to NB for MDR conformity assessment.
For information on the timeline extension click here.
Is your clinical evaluation MDR ready?
Updating clinical evaluation processes and documents to MDR requirements is a daunting task. From the major considerations, such as equivalence, to a myriad of minor details, clinical evaluation is indeed a heavy burden on the manufacturer in the transition. Even if you have updated your documents to MDR, latest guidance documents and also findings from audits and reviews should be incorporated to strengthen them. Clinical evaluation under MDR is an on-going, periodic process.
MDR Clinical Evaluation Data Management
Clinical evaluation under MDR is no longer one and done process. Periodic updates to clinical evaluation documents require a continuity in clinical evidence and the analysis. Data from one periodic update should seamlessly translate to the next revision without confusion or omission. Also, there has to be a systematic approach in clearly documenting the collection of clinical data and the decisions made in appraisal of the collected data.

MDR Clinical Evaluation Data Analysis
With MDR, the spotlight of clinical evaluation has been directed on safety and performance outcomes and benefit-risk ratio with a particular focus on quantitative analysis. Now, clinical data needs to be analyzed specifically to support each indication in a planned, quantitative approach. Starting with the clinical evaluation plan, it is important to clearly outline how the safety and performance outcomes are identified and quantified and how the clinical outcomes will inform benefit-risk analysis.
MDR Clinical Evaluation Intelligence
New Guidance on Equivalence in EU MDR Clinical Evaluation: Understanding MDCG 2023-7
The European Union Medical Device Regulation (EU MDR) has brought significant changes to the medical device industry. One area that has raised questions and uncertainties is the application of Articles 61(4)-(6) of the EU MDR on the requirement of clinical...
MDR Clinical Evaluation: A Guide to Systematic Literature Review
Conducting a systematic literature review is a crucial step in the clinical evaluation reporting process under EU MDR. It involves gathering and analyzing relevant clinical literature to support the safety and efficacy of medical devices. However, this process...
EU MDR Timeline Extension: what to do
The European Union Medical Device Regulation (EU MDR) has brought significant changes to the compliance timeline. With the transition timeline extension, manufacturers have an opportunity to ensure compliance with the new regulations. However, it's important to...
Deficiency Case: Insufficient Differentiation of Indications (Subpopulations)
In EU MDR, clinical data plays a crucial role in evaluating the safety and performance of the subject devices. However, deficiencies in clinical data can arise, leading to the need for further investigation and evidence collection. In this blog post, we will explore a...
Addressing Deficiencies in the Benefit-Risk Analysis
One crucial aspect of evaluating the safety and effectiveness of a device under MDR is the assessment of its benefit-risk ratio. The Clinical Evaluation Plan (CEP) plays a crucial role in establishing the process by providing a comprehensive analysis plan of the...
Deficiency in Establishing Equivalence
Welcome to our blog post where we delve into the world of clinical evaluation reports and the importance of addressing deficiencies. Today, we will focus on a specific deficiency related to equivalence and how it was successfully resolved. Join us as we explore...
Off-Label Use Data of an Equivalent Device
Welcome to our second post in the deficiency case series. In this post, we address an important aspect of clinical evaluation reports: the limitations of off-label use data in supporting equivalence for obese patients. In this post, we will explore why data from the...
MDR Clinical Evaluation: Beware of the Threshold
One important aspect of MDR clinical evaluation is the inclusion of Expected Performance Rates for safety and performance outcomes. These threshold values serve as benchmarks for evaluating the device's safety and performance. In this blog post, we will...
Clinical Evaluation and Deficiencies
With the implementation of EU MDR, clinical evaluation has taken the center stage with its significance elevated to be one of the crucial elements in a technical documentation. This means that the documentation of clinical evaluation is under heightened...